Zebrafish and Danionella Cerebrum behavior and calcium imagery
The rapid progress of modern neurosciences strongly relies on technological breakthroughs, both for stimulation, where a large effort is driven towards the production of complex (natural-like) sensory environments, and for neural recordings, where the main challenge is to obtain long-term measurements of the activity of a significant fraction of the neurons with single-cell resolution on intact animals. We aim at developing experimental tools to address both issues on a specific animal model, namely zebrafish larvae, and also, recently, Danionella translucida.
Zebrafish, which was originally developed as a model for embryo development, has recently emerged as a similarly important animal model for neurosciences. Zebrafish larvae exhibit stereotyped behavioral responses to various stimuli associated with several sensory modalities (e.g. visual, hydromechanical, auditive, gustatory, olfactory). However, most of the current researches focus on the visual system alone, owing to the lack of experimental protocols to deliver well-controlled stimuli to other modalities. In particular, very few data are currently available on the functioning and neural processing associated with the lateral line – the organ that mediates flow perception in fish and amphibians. We take advantage of the rapid development of microfluidic technologies to design devices that will allow for the precise delivering of complex spatio-temporal flow patterns onto the lateral line of a partially tethered zebrafish larva. Stimulation patterns can range from very localized stimuli to complex flows spanning a large portion of the animal's body and mimicking natural stimuli.
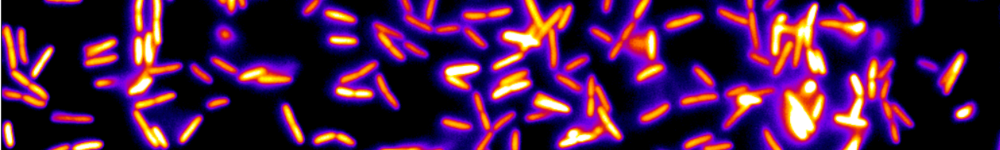
On the observation side, the rapid development of calcium imaging during the last two decades – tightly related to the progress of genetics – has led to remarkable improvements on the number of neurons whose activity can be simultaneously monitored. However, most current imaging setups (confocal and two-photon) exhibit intrinsic limitations – essentially related to their point-scanning nature – which impose a severe trade-off between the number of accessible neurons and the acquisition rate. Recently, Single-Plane Illumination Microscopy (SPIM) approaches, in which the optical sectioning is obtained through side-on illumination of the specimen by a thin laser-sheet, yielded spectacular progress for structural imaging during the first developmental stages of zebrafish larvae. We are adaptating this technique to 3D functional imaging of GCaMP transgenic larvae in order to reach simultaneous recordings at standard acquisition rates of an unprecedented amount of neurons. Here is a picture of a larva's brain obtained with SPIM:
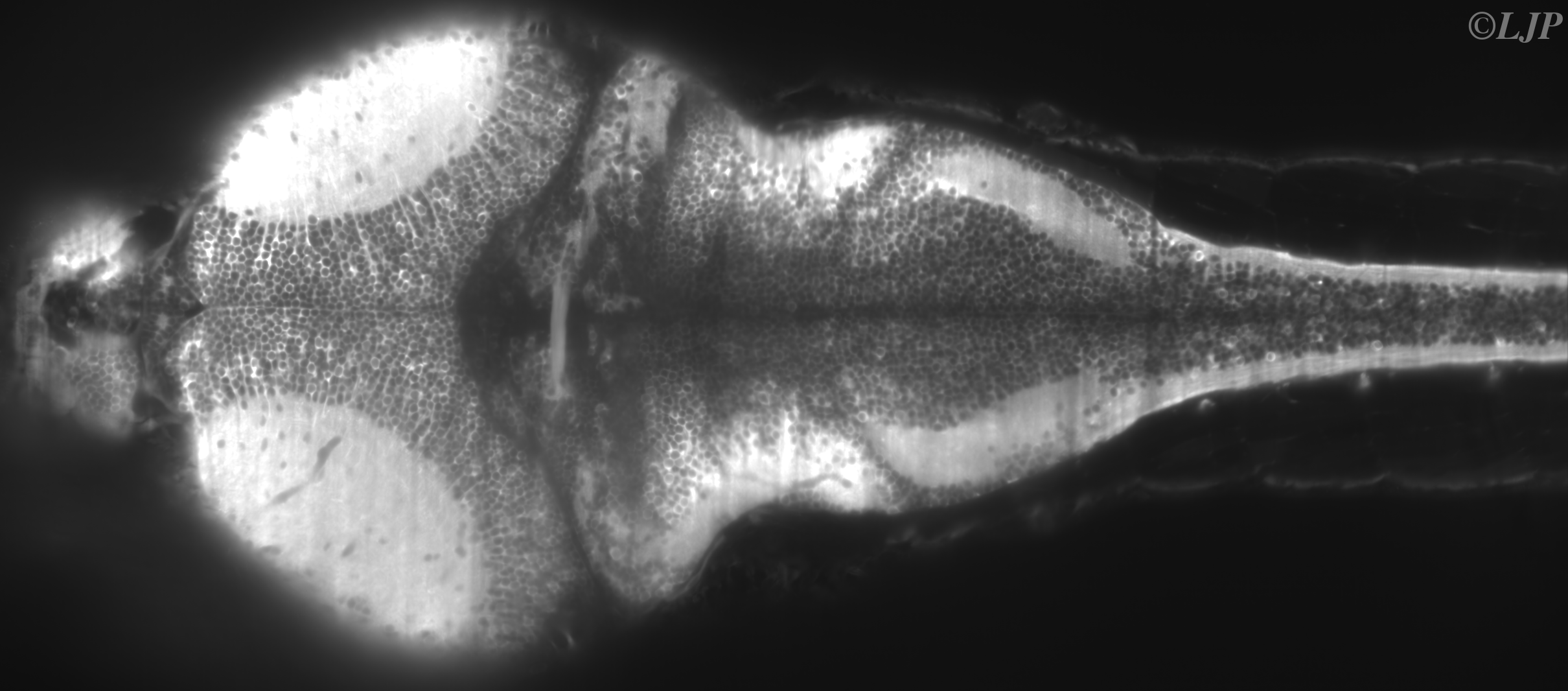
Figure 1 : Brain slice of a live and intact 6 d.p.f.GCaMP3 larva obtained by SPIM. Kernels appear in dark while somata are brighter. The image contains more than 5000 neurons.
Recordings the activity of a large fraction of the brain opens the way to new approaches in understanding how perceptive information is processed. In particular, large datasets allow for the search of brain-wide patterns via statistical correlations.
In the news
- "Observer en direct le fonctionnement du cerveau d’une larve de poisson-zèbre intacte" (French - Actualié de l'INP du CNRS, April 24, 2013)
- "Lumière sur les neurones" (French - Le Monde Science & techno, April 20, 2013)
PhD students (defense 2018->)
- Geoffrey Migault (2020)
- Sophia Karpenko (2020)
- Hugo Trentesaux (2020)
- Guillaume Le Goc (2021)
- Benjamin Gallois (2021)
- Julie Lafaye (2022)
- Natalia Beiza Canelo (2022)
- Hippolyte Moule (2023)
- Sharbatanu Chatterjee (2024)
- Matteo Dommanget Kott (2024)
- Leonardo Demarchi (2024)
- Monica Coraggioso (2024)
Post-docs (since 2018)
- Kristen Severie (2020)
- Thomas Panier (2016 - 2018)
- Thomas Pujol (2020)
- Marcus Ghosh (2021 - 2023)
- Antoine Hubert (2021 - present)
Master students (since 2018)
Master 1:
- 2018 Loann Collet
- 2018 Sahar Aghakhani
- 2019 Pierre Tapie
- 2020 Muntasir Callachand
- 2020 Emile Boré
- 2023 Elias Benyahia
Master 2:
- 2018 Julie Lafaye
- 2019 Patrik Turzak
- 2020 Matteo Dommaget-Kott
- 2020 Alexandre Nauleau
Programmes open-source
Related job openings
2024
2023
2023
Publications
2024
| ⊞ | Structure and individuality of navigation in zebrafish larvae - hal (Feb. 2024) |
2023
| ⊞ | Magnetic actuation of otoliths allows behavioral and brain-wide neuronal exploration of vestibulo-motor processing in larval zebrafish - Current Biology (Sep. 2023) |
| URL | Full text PDF | Bibtex | doi:https://doi.org/10.1016/j.cub.2023.05.026 |
| ⊞ | Random-access two-photon holographic optogenetic stimulation combined with brain-wide functional light-sheet imaging in larval zebrafish - Advances in Microscopic Imaging IV (Sep. 2023) |
| ⊞ | Multimodal units fuse-then-accumulate evidence across channels - BioRxiv (Jul. 2023) |
| ⊞ | A Versatile and Open Source One- and Two-Photon Light-Sheet Microscope Design - BioRxiv (Jul. 2023) |
|
|
|
Bibtex | doi:https://doi.org/10.1101/2023.07.10.548107 |
| ⊞ | Emergence of time persistence in an interpretable data-driven neural network model - ELIFE (Mar. 2023) |
|
|
|
Bibtex | doi:https://doi.org/10.7554/eLife.79541 |
| ⊞ | Neural assemblies uncovered by generative modeling explain whole-brain activity statistics and reflect structural connectivity - ELIFE (Mar. 2023) |
2022
| ⊞ | An analysis pipeline to compare explorative locomotion across fish species - STAR Protoc. (Nov. 2022) |
| ⊞ | A scalable assay for chemical preference of small freshwater fish - Frontiers in Behavioral Neuroscience (Sep. 2022) |
| URL | Full text PDF | Bibtex | doi:10.3389/fnbeh.2022.990792 |
| ⊞ | Evolutionary divergence of locomotion in two related vertebrate species - Cell Reports (Mar. 2022) |
| URL | Full text PDF | Bibtex | doi:10.1016/j.celrep.2022.110585 |
2021
| ⊞ | Thermal modulation of Zebrafish exploratory statistics reveals constraints on individual behavioral variability - BMC Biology (Sep. 2021) |
| URL | Full text PDF | Bibtex | doi:https://doi.org/10.1186/s12915-021-01126-w |
| ⊞ | Trans-inhibition of axon terminals underlies competition in the habenulo-interpeduncular pathway - Current Biology (Sep. 2021) |
| URL | Full text PDF | Bibtex | doi:10.1016/j.cub.2021.08.051 |
| ⊞ | FastTrack: An open-source software for tracking varying numbers of deformable objects - PLOS Computational Biology (Feb. 2021) |
| URL | Full text PDF | Bibtex | doi:10.1371/journal.pcbi.1008697 |
2020
| ⊞ | From behavior to circuit modeling of light-seeking navigation in zebrafish larvae - eLife (Jan. 2020) |
| URL | Full text PDF | Bibtex | doi:10.7554/eLife.52882 |
2019
| ⊞ | A Semi-Automatic Dispenser for Solid and Liquid Food in Aquatic Facilities - Zebrafish (Aug. 2019) |
| URL | Full text PDF | Bibtex | doi:10.1089/zeb.2019.1733 |
2018
| ⊞ | Whole-Brain Calcium Imaging during Physiological Vestibular Stimulation in Larval Zebrafish - Current Biology (Nov. 2018) |
| URL | Full text PDF | Bibtex | doi:https://doi.org/10.1016/j.cub.2018.10.017 |
2017
| ⊞ | Sensorimotor computation underlying phototaxis in zebrafish - Nature Communication (Sep. 2017) |
|
|
Full text PDF | Bibtex | doi:10.1038/s41467-017-00310-3 |
| ⊞ | Blind sparse deconvolution for inferring spike trains from fluorescence recordings - BioArxiv (Jun. 2017) |
|
|
Full text PDF | Bibtex | doi:10.1101/156364 |
2016
| ⊞ | A 2D virtual reality system for visual goal-driven navigation in zebrafish larvae - Scientific Reports (Sep. 2016) |
| URL | Full text PDF | Bibtex | doi:10.1038/srep34015 |
| ⊞ | Rheotaxis of Larval Zebrafish: Behavioral Study of a Multi-Sensory Process - Frontiers in System Neuroscience (Feb. 2016) |
| URL | Full text PDF | Bibtex | doi:10.3389/fnsys.2016.00014 |
2015
| ⊞ | A microfluidic device to study neuronal and motor responses to acute chemical stimuli in zebrafish - Scientific Reports (Jul. 2015) |
| URL | Full text PDF | Bibtex | doi:10.1038/srep12196 |
| ⊞ | Whole-brain functional imaging with two-photon light-sheet microscopy - Nature Methods (Apr. 2015) |
| URL | Full text PDF | Bibtex | doi:10.1038/nmeth.3371 |
2013
| ⊞ | Fast functional imaging of multiple brain regions in intact zebrafish larvae using Selective Plane Illumination Microscopy - Frontiers in Neural Circuits (Apr. 2013) |
| URL | Full text PDF | Bibtex | doi:10.3389/fncir.2013.00065 |