DEC 2018
Florence Bansept has the pleasure to invite you to her PhD defense that will take place on Wednesday, the 5th of December at 10AM in amphithéâtre Charpak (level SB, at the bottom of tower 22, 4 place Jussieu, Paris 5ème), where she will present her work on
Biophysical modeling of bacterial population dynamics and the immune response in the gut,
and to the "pot" that will follow in the seminar room of Laboratoire Jean Perrin (tower 32-33, 5th floor).
The defense will be held in English in front of the following jury:
- M. Raphaël Voituriez, Directeur de thèse
- M. Bahram Houchmandzadeh, Rapporteur
- Mme. Agnese Seminara, Rapportrice
- Mme. Aleksandra Walczak, Examinatrice
- Mme. Silvia De Monte, Examinatrice
- M. Andrea Parmeggiani, Examinateur
- Mme. Emma Wetter-Slack, Invitée
- Mme. Claude Loverdo, Invitée
Abstract
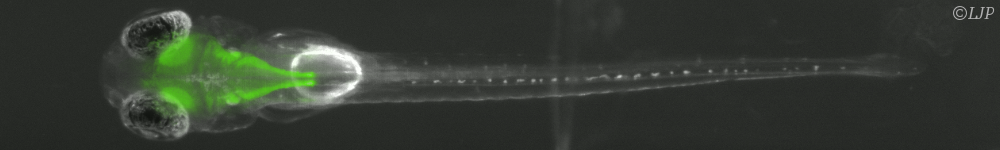
The first part of this thesis focuses on the colonization dynamics of a bacterial population in early infection of the gut. The aim is to infer biologically relevant parameters from indirect data. We discuss the optimal observable to characterize the variability in genetic tags distributions. In a first one-population model, biological arguments and inconsistencies between several experimental observables lead to the study of a second model with two-subpopulations replicating at different rates. As expected, this model allows for broader possibilities in observables combination, even though no clear conclusion can be drawn as to a data set on Salmonella in mice.
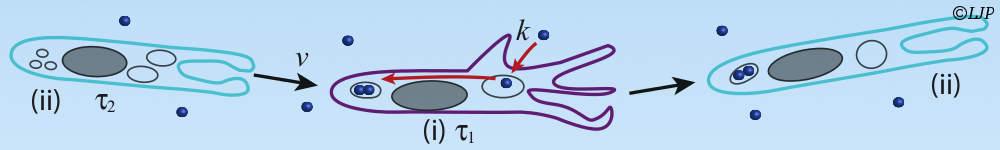
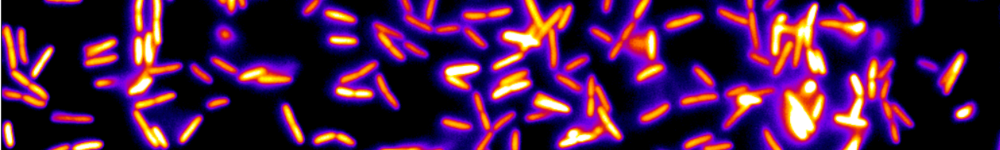
The second part concerns the mechanisms that make the immune response effective. The main effector of the immune system in the gut, IgA (an antibody), enchains daughter bacteria in clonal clusters upon replication. Our model predicting the ensuing reduction of diversity in the bacterial population contributes to evidence this phenomenon, called “enchained growth”. Inside the host, the interplay of cluster growth and fragmentation results in preferentially trapping faster-growing and potentially noxious bacteria away from the epithelium, which could be a way for the immune system to regulate the microbiota composition. At the scale of the hosts population, in the context of evolution of antibiotic resistance, if bacteria are transmitted via clonal clusters, the probability to transmit a resistant bacteria is reduced in immune populations. Thus we use statistical physics tools to identify some generic mechanisms in biology.