Accueil
>
Séminaires
>
A blueprint for actomyosin assembly and disassembly during pulsed contractions: imaging single molecules in a developing embryo
|
A blueprint for actomyosin assembly and disassembly during pulsed contractions: imaging single molecules in a developing embryo
Par François Robin (Institut de Biologie Paris-Seine)
Le 30 Mars 2015 à 11h00 - seminar room LJP (tower 32, 5th floor)
|
Résumé
Actomyosin-based pulsed contractions are a widespread and highly dynamic form of self-organized contractility that underlies numerous morphogenetic events during early development. However the mechanisms that govern pulsed contractions remain poorly understood.
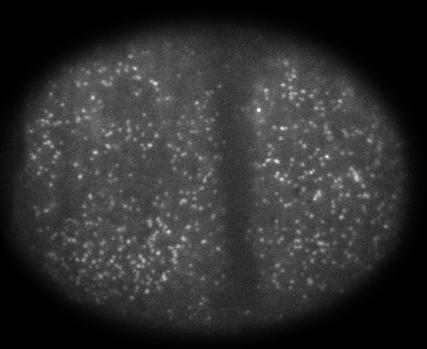
We combined single molecule imaging and single particle tracking analysis to quantify actomyosin assembly/disassembly in relation to the onset and terminations of pulsed contractions. We found that sharp increases in assembly/recruitment rates and stability of both F-actin and Myosin II precede the onset of contraction by ~ 6 second. These data rule out models for the initiation of pulsed contractions in which local contraction concentrates factors that promote further contraction, and instead suggest mechanisms in which phasic modulation of assembly/disassembly drive initiation and termination of pulsed contractions. To address this further, we measured the relative timing of appearance/disappearance for different components of pulsed contractions. Strikingly, F-actin, Myosin and the multivalent scaffold Annillin-1 accumulate and disappear with nearly identical timing during each pulsed contraction. However the active form of their common upstream regulator Rho-1 appears before all three and well before the onset of observable contraction, suggesting that pulsed accumulation of active Rho is a key timer for pulsed contractions. Finally, consistent with these observations, neither Myosin nor Anillin are required for pulsed accumulations of Rho-1.
In conclusion, our data show that pulsed contractions do not arise through contractile instabilities, tensionbased stabilization or dynamical clustering of cortical actomyosin. Instead, we show that pulsed contractions are preceded and driven by local, autocatalytic pulses of activated RhoA, independently of Myosin and Anillin.