|
Multi-protein complexes: functions and constraints
Par Anne-Florence Bitbol (Princeton University)
Le 21 Novembre 2014 à 11h00 - Salle de réunion du LJP (tour 32, 5ème étage)
|
Résumé
(Lewis-Sigler Institute for Integrative Genomics and Department of Physics, Princeton University)
Self-assembled multi-protein complexes play key roles in cells, e.g., as molecular motors, enzymes, channels, and receptors. Functional interactions between proteins forming these complexes impose constraints on their evolution, on their abundances and on their shapes. In turn, basic features of the assembly process can be harnessed in biological function. We investigate theoretically some aspects of this fundamental link between functions and constraints in multi-protein complexes, from the population scale to the molecular scale.
Proteins that form a complex are evolutionarily coupled by the need to preserve functional interactions. This coupling can lead to the existence of fitness valleys, which hinder further evolution. Fitness valley or plateau crossing can be facilitated by specific population structures. Using a minimal model, we quantitatively determine when, and to what extent, population subdivision accelerates valley and plateau crossing.
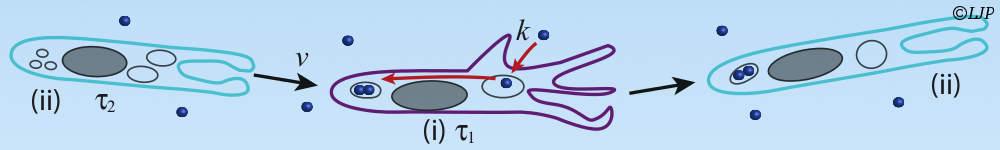
At the cellular scale, function can impose constraints on the abundances of proteins. E. coli chemotaxis is a model for signal transduction. However, the proteins involved are expressed in surprisingly large numbers. We trace the need for this large number of chemotaxis proteins to the specific need of the chemotaxis system for fast response, and to the requirements of self-assembly. Besides, the abundance of all the chemotaxis proteins significantly increases in poorer medium. We show that the gain of the pathway increases upon concerted over-expression of the chemotaxis proteins, which may be beneficial in poor nutritional conditions.
At the molecular scale, the shapes of proteins are constrained by the need for maintaining specific interactions while avoiding non-specific ones. We show that real protein shapes feature a great diversity. This makes spurious shape complementarity very unlikely, thus reducing the risk of non-specific interactions.
Hence, functional interactions impose constraints on proteins at multiple levels. Fundamental features of the self-assembly process, such as the possible existence of nucleation barriers, can in turn serve a particular biological function. The enzyme CTP synthetase forms long polymers in cells. Our experimental collaborators have shown that this polymerization is coupled to negative feedback by the product of the enzyme. We show that coupling enzyme activity to polymerization with a nucleation barrier enables ultrasensitive enzymatic regulation.