|
Mecano-transduction : From a single cell (µm), in vitro, to a single embryo (mm), in vivo.
Par Demostène Mitrossilis (Institut Curie), candidat poste MdC
Le 10 Février 2014 à 11h00 - Salle de réunion du LJP (5-31)
|
Résumé
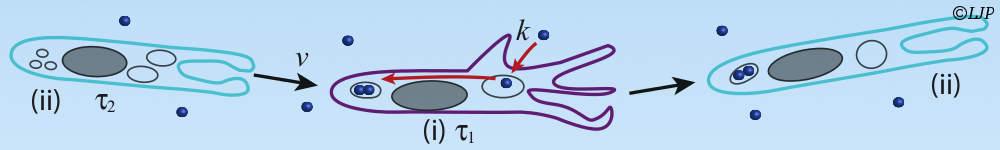
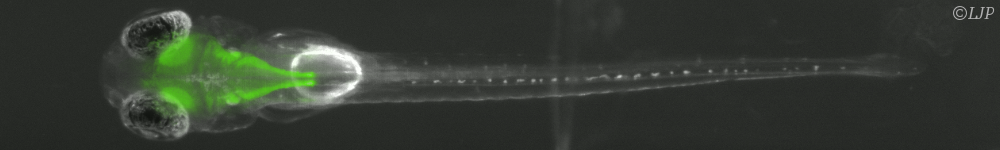
Mechanics occurs in many phenomenon of living matter in nature. Mechanical cues generated by developmental strain deformation were found to activate major developmental biochemical events, in living embryos, including early anterior endoderm specification, early mesoderm differentiation joint bone differentiation. Single cells are also able to adapt their activity to mechanical environment, as rigidity. Rigidity is able to be as a control parameter of differentiation of single stem cell. However, cell response to stiffness is mainly thought to be initiated by the deformation of adhesion complexes under applied force. We were able to measure the traction force as well as the speed of shortening of isolated myoblasts deflecting micro-plates of variable stiffness. The rate of force generation increased with increasing stiffness and followed a Hill force–velocity relationship. Hence, cell response to stiffness was similar to muscle adaptation to load, reflecting the force-dependent kinetics of myosin binding to actin. In order to determine whether cell response was triggered by stiffness or force, we have developed a unique method allowing us to tune, in real time, the effective stiffness experienced by a single living cell in a uniaxial traction geometry. In these conditions, the rate of traction force buildup dF/dt was adapted to stiffness in less than 0.1 s. This integrated fast response was unambiguously triggered by stiffness, and not by force. It suggests that early cell response could be mechanical in nature. In addition, biochemical patterning and biomechanical morphogenesis represent the major components of embryonic development regulating the shaping of living organisms. The reminiscent question of the translation of a biochemical genetic information into a 3 dimension tissue functional morphology begins to be understood in different developmental contexts. The reciprocal role of the mechanical cues developed by biomechanical morphogenetic movements, on the activation of developmental biochemical pathways involved in both the differentiation and the morphogenetic movements trigger, is emerging as a key feature of embryonic development regulation. However, the demonstration of the requirement of the sna regulated single cell pulsations in the mechanical activation the Fog dependent developmental biochemical pathway leading to apical stabilization of Myo-II, required the application of physiologically relevant strain deformations quantitatively mimicking the morphogenetic movements lacking in the sna genetic mutant context, in a full biochemical independent way, in vivo.