2024
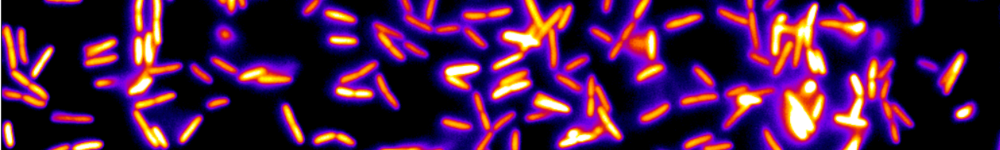
Multispecies bacterial biofilm using Raman micro-spectroscopy.
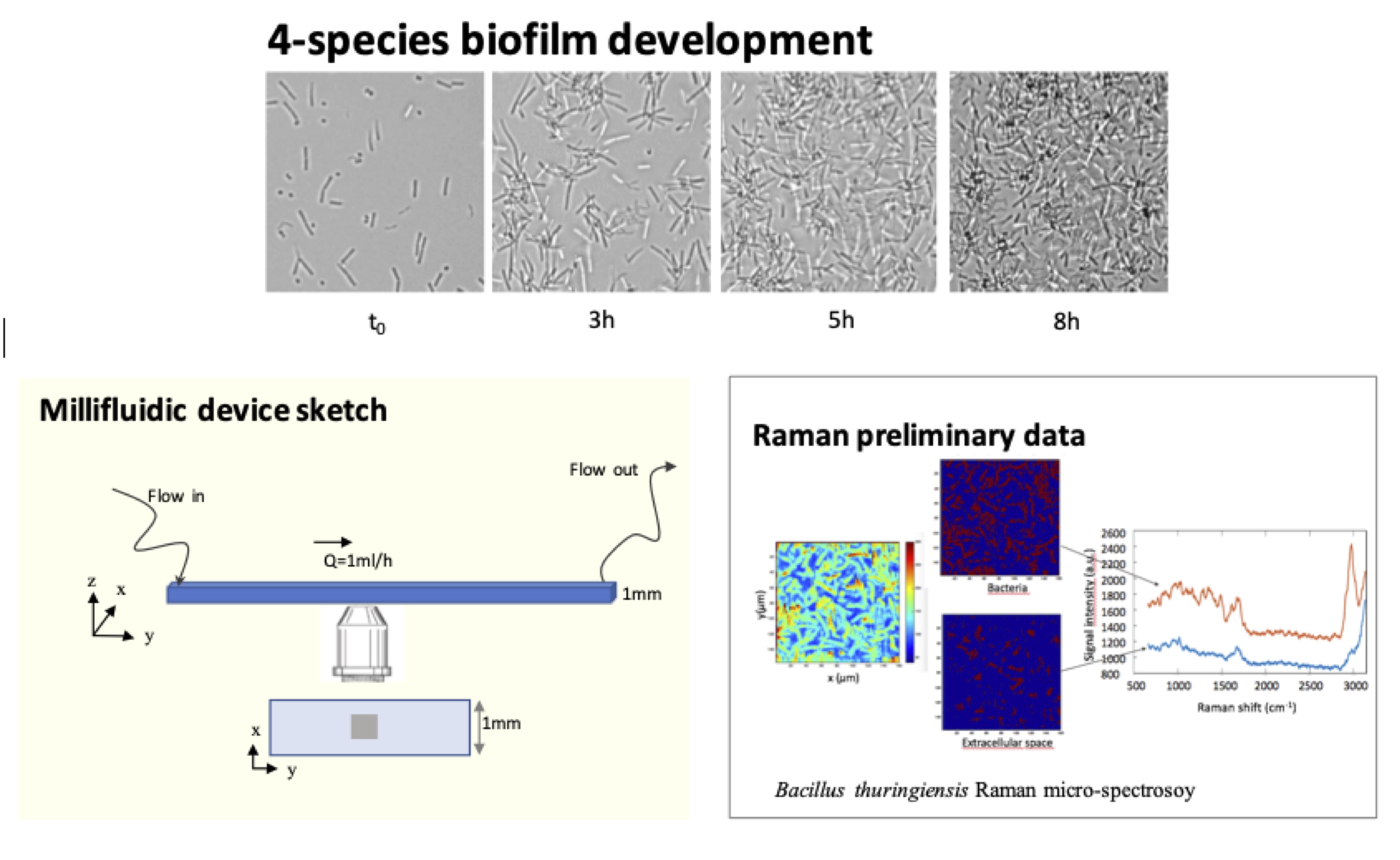
The extracellular matrix is the polymer soft architecture of a bacterial biofilm. It physically stabilizes the cells and their offsprings, giving rise to a 3D living material expressing emergent properties. How this organ assembles in the context of a multi-sepcies biofilm is completely unknown. To gain insights into this question we aim at implementing a recent innovation of micro-Raman spectroscopy — the compressive micro-Raman— on a 4-species model biofilm growing under flow of nutrients in a millifluidic channel. (More details here)
The project will be co-supervised by Nelly Henry - Laboratoire Jean Perrin (CNRS, SU) and Hilton B. de Aguiar (LKB, CNRS, ENS). Paris 5e, France .
Candidate profile: Main background in physics or physico-chemistry with a strong interest in biology. A master 2 student at the interface of these disciplines would be highly appreciated. Yet a strong motivation for biological systems will also be favorably considered for a candidate with no background in biology.
Contact: nelly.henry@sorbonne-universite.fr and h.aguiar@phys.ens.fr
References:
1. P. Thomen, J. D. P. Valentin, A. F. Bitbol, N. Henry, Spatiotemporal pattern formation in E.coli biofilms explained by a simple physical energy balance. Soft Matter 16, 494-504 (2020)
2. Monmeyran et al., The inducible chemical-genetic fluorescent marker FAST outperforms classical fluorescent proteins in the quantitative reporting of bacterial biofilm dynamics. Sci Rep 8, 10336 (2018)
3. P. Thomen et al., Bacterial biofilm under flow: First a physical struggle to stay, then a matter of breathing. PLoS ONE 12, e0175197 (2017)
4. O. Galy et al., Mapping of bacterial biofilm local mechanics by magnetic microparticle actuation. Biophysical journal 5, 1400-1408 (2012).
5. S. H. Donaldson Jr., H. B. de Aguiar. Molecular Imaging of Cholesterol and Lipid Distributions in Model Membranes. J. Phys. Chem. Lett. 9 1528 (2018)
6. B. Sturm, F. Soldevila, E. Tajahuerce, S. Gigan, H. Rigneault, H. B. de Aguiar. High-sensitivity high-speed compressive spectrometer for Raman imaging. ACS Photonics 6, 1409–1415 (2019)
7. F. Soldevila, J. Dong, E. Tajahuerce, S. Gigan, H. B. de Aguiar. Fast compressive Raman bio-imaging via matrix completion. Optica 6, 341–346 (2019)