|
Making and Folding Colloidomers
Par Jasna Brujic (NYU)
Le 9 Novembre 2021 à 11h00 - Salle de séminaires 5ème étage, Tour 32-33
|
Résumé
Making and Folding Colloidomers By Design
Angus McMullen, Maitane Munoz Basagoiti, Zorana Zeravcic, Jasna Brujic
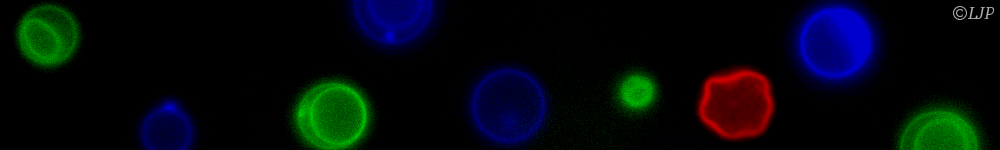
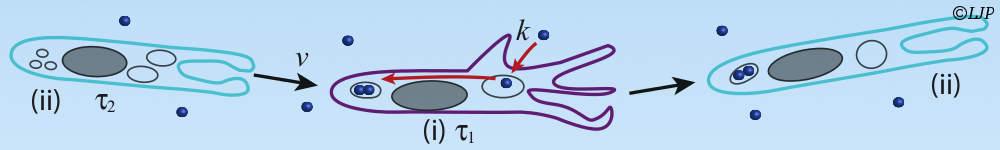
Protein folding is an efficient mechanism used by biology to make molecules with a particular design from a given amino acid sequence. To implement the same strategy in materials science, here we self-assemble N=3-12 colloidal droplets into freely-jointed linear chains, colloidomers, which then fold into specific structures via programmable secondary interactions. Anfinsen's dogma argues that proteins fold into a unique thermodynamically stable structure, along a funnel-like, smooth landscape to avoid kinetic traps. On the colloidal length scale, equilibrium structures are difficult to achieve on experimental timescales due to the vastness of possible configurations. Instead, nonequilibrium temperature quenches are here optimized using folding trees to achieve the desired fold fast. Surprisingly, simple hetero-colloidomer (ABABA) sequences with only one or two secondary interactions are sufficient to achieve unique rigid or flexible folds as their final goal. We thus demonstrate key concepts needed for folding, including the uniqueness of the folded state, its kinetic accessibility, and robustness against mutations in the interaction matrix. Even though our experiments and simulations explore only downhill folding, we uncover principles of organization that are reminiscent of those proposed in biological proteins.